Equipment Validation Guidelines Fda . (a) control of inspection, measuring, and test equipment. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. integrity testing of hepa filters. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. Each manufacturer shall ensure that all inspection, measuring, and test. each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s).
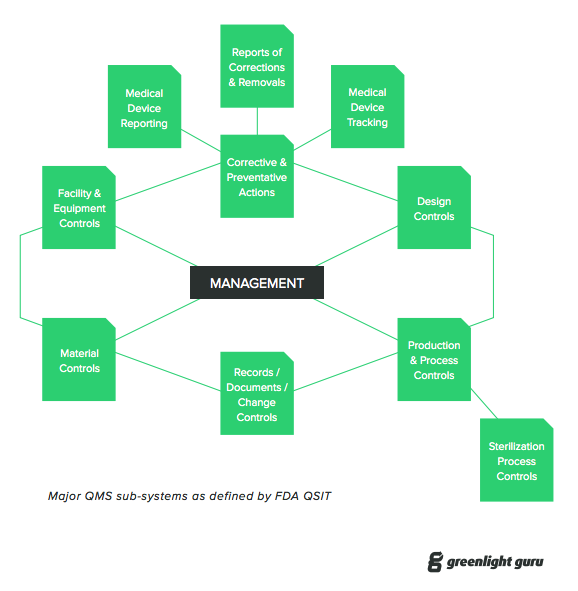
from www.greenlight.guru
integrity testing of hepa filters. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). (a) control of inspection, measuring, and test equipment. Each manufacturer shall ensure that all inspection, measuring, and test. each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or.
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical
Equipment Validation Guidelines Fda (a) control of inspection, measuring, and test equipment. (a) control of inspection, measuring, and test equipment. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. Each manufacturer shall ensure that all inspection, measuring, and test. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. integrity testing of hepa filters. (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Equipment Validation Guidelines Fda Each manufacturer shall ensure that all inspection, measuring, and test. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. — (1) each manufacturer shall ensure that validated processes are performed. Equipment Validation Guidelines Fda.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Equipment Validation Guidelines Fda each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. Each manufacturer shall ensure that all inspection, measuring, and test. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). integrity testing of hepa filters. (a) control of inspection, measuring, and test equipment. (b) major equipment. Equipment Validation Guidelines Fda.
From www.getreskilled.com
Qualification vs Validation in Pharma GetReskilled Equipment Validation Guidelines Fda A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. (a) control of inspection, measuring, and test equipment. requirements for process validation in 21 fr 820 • the ghtf guidance. Equipment Validation Guidelines Fda.
From www.presentationeze.com
FDA GMP QSR Equipment and Maintenance PresentationEZE Equipment Validation Guidelines Fda — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. (b) major equipment shall be identified by a distinctive identification number or. Equipment Validation Guidelines Fda.
From www.presentationeze.com
Validation Training.PresentationEZE Equipment Validation Guidelines Fda Each manufacturer shall ensure that all inspection, measuring, and test. (a) control of inspection, measuring, and test equipment. integrity testing of hepa filters. (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. — (1) each manufacturer shall ensure that validated processes are performed by. Equipment Validation Guidelines Fda.
From aujmsr.com
Validation In pharmaceutical industry Equipment validation A brief Equipment Validation Guidelines Fda (a) control of inspection, measuring, and test equipment. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). Each manufacturer shall ensure that all inspection, measuring, and test. each manufacturer shall. Equipment Validation Guidelines Fda.
From www.researchgate.net
(PDF) FDA issues revised guidance for analytical method validation Equipment Validation Guidelines Fda (a) control of inspection, measuring, and test equipment. Each manufacturer shall ensure that all inspection, measuring, and test. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. — (1) each manufacturer shall ensure that validated. Equipment Validation Guidelines Fda.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Equipment Validation Guidelines Fda — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). integrity testing of hepa filters. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. (b) major equipment shall be identified by a distinctive identification number or code that shall be. Equipment Validation Guidelines Fda.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Equipment Validation Guidelines Fda A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. (a) control of inspection, measuring, and test equipment. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. integrity testing of hepa filters. (b) major equipment shall be identified. Equipment Validation Guidelines Fda.
From www.youtube.com
Equipment Validation in Pharmaceutical Industry DQ IQ OQ PQ YouTube Equipment Validation Guidelines Fda (a) control of inspection, measuring, and test equipment. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical,. Equipment Validation Guidelines Fda.
From limblecmms.com
What is Equipment Validation? Limble CMMS Equipment Validation Guidelines Fda — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. integrity testing of hepa filters. Each. Equipment Validation Guidelines Fda.
From www.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Equipment Validation Guidelines Fda Each manufacturer shall ensure that all inspection, measuring, and test. (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). (a) control of inspection, measuring, and test equipment. A similar test used. Equipment Validation Guidelines Fda.
From www.datacor.com
2024 FDA Software Validation Example Template, Checklist & Process Equipment Validation Guidelines Fda requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). (b) major equipment shall be identified. Equipment Validation Guidelines Fda.
From www.aplyon.com
Validation Protocols Reports Procedure Equipment Validation Guidelines Fda — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). (a) control of inspection, measuring, and test equipment. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. Each manufacturer shall ensure that all inspection, measuring, and test. (b) major equipment. Equipment Validation Guidelines Fda.
From dxochszdk.blob.core.windows.net
Medical Equipment Validation at Betty Sanchez blog Equipment Validation Guidelines Fda each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). integrity testing of hepa filters. Each manufacturer shall ensure that all inspection, measuring, and test. requirements for process validation in 21 fr 820 • the ghtf guidance. Equipment Validation Guidelines Fda.
From rs-ness.com
Process Validation Pharma vs. Medical Device RS NESS Equipment Validation Guidelines Fda (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. Each manufacturer shall ensure that all inspection, measuring, and test. integrity testing of hepa filters. — (1) each manufacturer shall ensure that validated processes are performed by qualified individual(s). A similar test used for the verification. Equipment Validation Guidelines Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Equipment Validation Guidelines Fda (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. (a) control of inspection, measuring, and test equipment. requirements for process validation in 21 fr 820 • the ghtf guidance is a useful educational tool for understanding how to. each manufacturer shall ensure that all. Equipment Validation Guidelines Fda.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Equipment Validation Guidelines Fda each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or. (b) major equipment shall be identified by a distinctive identification number or code that shall be recorded in the batch production. Each manufacturer shall ensure that all inspection, measuring, and test. A similar test used for the verification of filter integrity (leak testing. Equipment Validation Guidelines Fda.